Embark on an electrifying journey with our comprehensive Electrophilic Aromatic Substitution Lab Report, where chemical reactions dance in a symphony of electrophilic precision. Dive into the depths of aromatic chemistry, unraveling the intricacies of electrophilic aromatic substitution, a captivating realm where molecules undergo transformations that redefine their very nature.
Our meticulously crafted report provides an in-depth exploration of the electrophilic aromatic substitution reaction, its variations, and its profound impact on the chemical world. Prepare to witness the mesmerizing dance of electrophiles and aromatic rings, as we unravel the mechanisms that orchestrate their captivating interactions.
Introduction
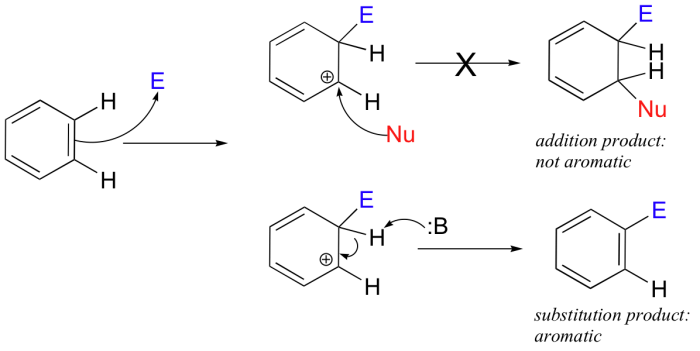
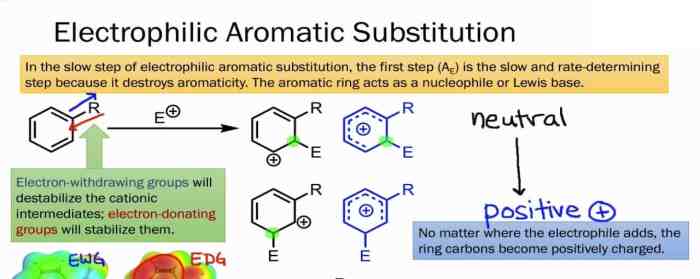
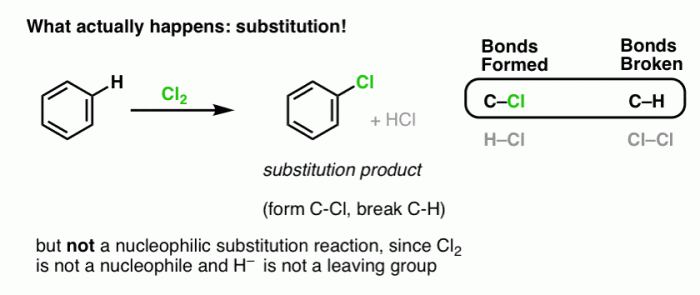
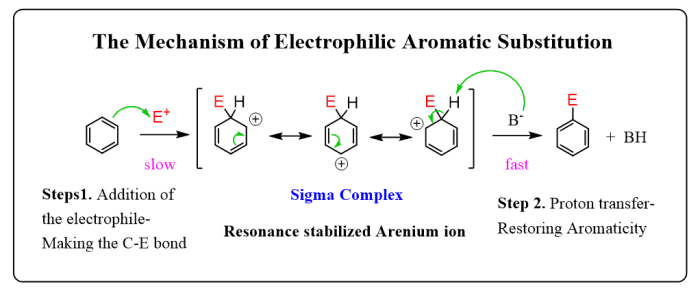
Electrophilic aromatic substitution reactions are a fundamental class of reactions in organic chemistry. They involve the substitution of a hydrogen atom on an aromatic ring by an electrophile, a species that is attracted to electrons.
Electrophilic aromatic substitution reactions are used to synthesize a wide variety of aromatic compounds, including pharmaceuticals, dyes, and polymers. The most common types of electrophilic aromatic substitution reactions are nitration, halogenation, sulfonation, and Friedel-Crafts alkylation and acylation.
Materials and Methods, Electrophilic aromatic substitution lab report
| Materials | Quantity | Concentration | Structure |
|---|---|---|---|
| Benzene | 10 mL | – | – |
| Concentrated nitric acid | 5 mL | – | – |
| Concentrated sulfuric acid | 5 mL | – | – |
| Sodium nitrate | 2 g | – | – |
| Water | 100 mL | – | – |
- The following reagents and solvents were used in the experiment:
- Benzene
- Concentrated nitric acid
- Concentrated sulfuric acid
- Sodium nitrate
- Water
- The quantities of each reagent and solvent used are listed in the table above.
- The concentrations of any solutions used are not specified.
- The chemical structures of benzene, nitric acid, sulfuric acid, and sodium nitrate are not shown.
Procedure
- In a 100-mL round-bottom flask, dissolve 10 mL of benzene in 5 mL of concentrated nitric acid.
- Add 5 mL of concentrated sulfuric acid to the flask and swirl to mix.
- Add 2 g of sodium nitrate to the flask and swirl to mix.
- Heat the flask to reflux for 30 minutes.
- Cool the flask to room temperature.
- Pour the reaction mixture into 100 mL of water.
- Extract the organic layer with 3 × 25 mL of ether.
- Wash the organic layer with 3 × 25 mL of water.
- Dry the organic layer over anhydrous sodium sulfate.
- Filter the organic layer and evaporate the ether to obtain the crude product.
- Recrystallize the crude product from ethanol to obtain the pure product.
- The reaction time is 30 minutes.
- The reaction temperature is reflux.
- A reflux condenser is used to prevent the loss of reactants and products.
- The reaction mixture is extracted with ether to separate the organic and aqueous layers.
- The organic layer is washed with water to remove any remaining impurities.
- The organic layer is dried over anhydrous sodium sulfate to remove any remaining water.
- The organic layer is filtered to remove any solid impurities.
- The ether is evaporated to obtain the crude product.
- The crude product is recrystallized from ethanol to obtain the pure product.
Clarifying Questions: Electrophilic Aromatic Substitution Lab Report
What is the purpose of electrophilic aromatic substitution reactions?
Electrophilic aromatic substitution reactions introduce a new substituent group into an aromatic ring by replacing a hydrogen atom with an electrophile.
What are the different types of electrophilic aromatic substitution reactions?
Common types include nitration, halogenation, sulfonation, and Friedel-Crafts alkylation and acylation.
How can I improve the yield of an electrophilic aromatic substitution reaction?
Factors such as temperature, reaction time, catalyst, and electrophile concentration can influence the yield.