Determine the oxidation state of nitrogen in rbno2 – The determination of oxidation states, exemplified by the case of nitrogen in RbNO2, is a fundamental aspect of chemistry that unveils the intricate electronic configurations of compounds. This comprehensive guide delves into the concept of oxidation states, providing a step-by-step approach to determining the oxidation state of nitrogen in RbNO2.
Through an exploration of its chemical structure, properties, and the application of oxidation state rules, we unravel the factors that influence the oxidation state of nitrogen and its implications in various chemical contexts.
Oxidation State of Nitrogen in RbNO2: Determine The Oxidation State Of Nitrogen In Rbno2

In chemistry, the oxidation state of an atom refers to the hypothetical charge it would have if all its bonds to different atoms were ionic. It provides valuable insights into the chemical behavior and bonding characteristics of elements and compounds.
RbNO2 Compound
Rubidium nitrite (RbNO2) is an inorganic compound consisting of rubidium (Rb), nitrogen (N), and oxygen (O) atoms. It has a chemical structure of Rb+ and NO2-. The NO2- ion is a nitrite ion, which is a resonance hybrid of two equivalent Lewis structures.
RbNO2 is a white to pale yellow crystalline solid that is soluble in water. It is a strong oxidizing agent and can react with reducing agents to form rubidium oxide (Rb2O) and nitrogen dioxide (NO2).
Oxidation State Determination
To determine the oxidation state of nitrogen in RbNO2, we need to consider the oxidation states of the other elements in the compound.
Rubidium (Rb) is an alkali metal and always has an oxidation state of +1.
Oxygen (O) typically has an oxidation state of -2 in most compounds.
Let x be the oxidation state of nitrogen in RbNO2.
Using the rule that the sum of the oxidation states of all atoms in a neutral compound is zero, we can write the equation:
“`+1 + x + (-2) = 0“`
Solving for x, we get:
“`x = +3“`
Therefore, the oxidation state of nitrogen in RbNO2 is +3.
Oxidation State Rules
There are several general rules for determining oxidation states:
- The oxidation state of an element in its elemental form is 0.
- The oxidation state of a monatomic ion is equal to its charge.
- The oxidation state of hydrogen is +1 when bonded to nonmetals and -1 when bonded to metals.
- The oxidation state of oxygen is -2 in most compounds.
- The oxidation state of a group 1 metal (e.g., Na, K, Rb) is +1.
- The oxidation state of a group 2 metal (e.g., Ca, Mg, Ba) is +2.
In the case of RbNO2, the oxidation state of nitrogen is determined by applying these rules and considering the oxidation states of the other elements in the compound.
Comparison with Other Nitrogen Compounds
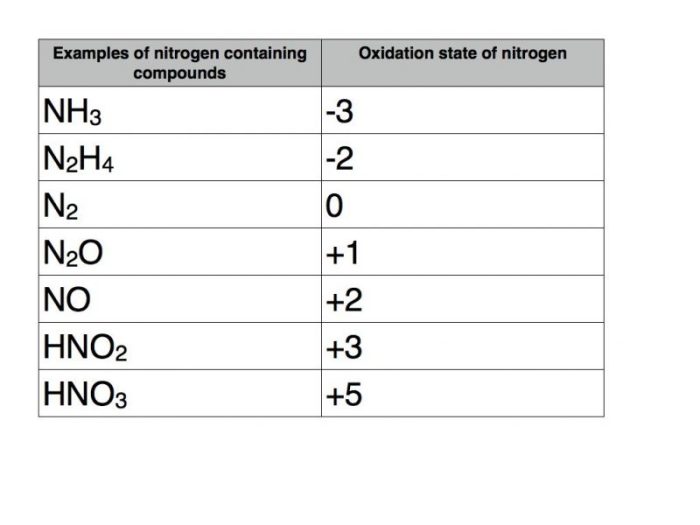
The oxidation state of nitrogen in RbNO2 (+3) is different from that in other nitrogen compounds, such as ammonia (NH3) and nitrite ion (NO2-).
In NH3, nitrogen has an oxidation state of -3, while in NO2-, it has an oxidation state of +3. This variation in oxidation state reflects the different bonding and electronic structures of these compounds.
The oxidation state of nitrogen is influenced by factors such as the type of bonds it forms, the electronegativity of the atoms it is bonded to, and the overall charge of the compound.
Applications of Oxidation State, Determine the oxidation state of nitrogen in rbno2
Determining oxidation states is crucial in chemistry for several reasons:
- It helps predict the chemical behavior of elements and compounds.
- It aids in balancing chemical equations.
- It provides insights into the electronic structure and bonding of molecules.
- It is used in redox reactions to determine the changes in oxidation states of reactants and products.
FAQs
What is the oxidation state of nitrogen in RbNO2?
+3
How do you determine the oxidation state of nitrogen in RbNO2?
By assigning oxidation states to the other atoms in the compound and then using the overall charge of the compound to determine the oxidation state of nitrogen.
What are the applications of oxidation state determination?
Oxidation state determination is used in a variety of applications, including predicting the reactivity of compounds, understanding reaction mechanisms, and determining the electronic structure of compounds.