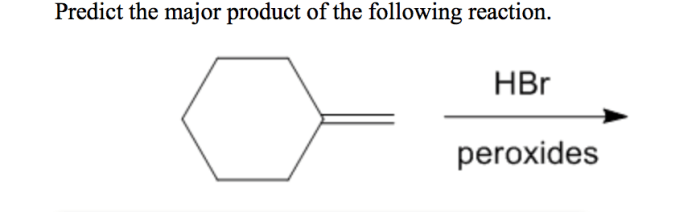
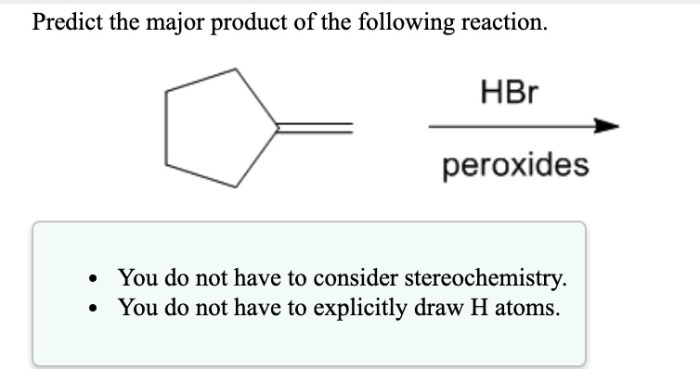
Predict the major product of the following reaction. hbr peroxides – Predicting the major product of the following reaction. HBr peroxides are an essential aspect of organic chemistry, enabling the selective formation of various products. This article delves into the intricacies of HBr peroxide reactions, exploring their mechanisms, influencing factors, and practical applications.
HBr peroxides, characterized by their unique chemical structure and properties, serve as radical initiators, triggering a cascade of reactions that ultimately lead to the formation of specific products. Understanding the factors governing these reactions, such as the stability of radicals and the regio- and stereoselectivity of the process, is crucial for predicting the major product.
Overview of HBr Peroxides
HBr peroxides are a class of organic compounds that contain a peroxide bond (O-O) adjacent to a bromine atom (Br). They are typically colorless or pale yellow liquids or solids with pungent odors. HBr peroxides are highly reactive and can undergo a variety of reactions, including radical initiations, oxidations, and additions.
Reaction Mechanisms: Predict The Major Product Of The Following Reaction. Hbr Peroxides
HBr peroxides react via a radical chain mechanism. In this mechanism, the peroxide bond homolytically cleaves to form two bromine radicals. These radicals can then react with other molecules to initiate a chain of radical reactions.
Factors Influencing the Formation of Major Products
The major product of an HBr peroxide reaction is determined by a number of factors, including:
- The structure of the starting material
- The reaction conditions
- The presence of other reagents
Predicting Major Products, Predict the major product of the following reaction. hbr peroxides
To predict the major product of an HBr peroxide reaction, it is important to consider the factors listed above. In general, the major product will be the most stable product that can be formed under the reaction conditions.
Examples and Applications
HBr peroxides are used in a variety of applications, including:
- As radical initiators in polymerization reactions
- As oxidizing agents in organic synthesis
- As bleaching agents in the textile industry
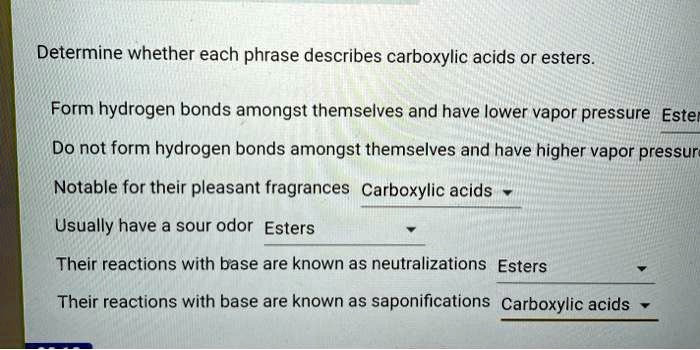
Helpful Answers
What are the key factors influencing the formation of major products in HBr peroxide reactions?
The stability of radicals, regioselectivity, and stereoselectivity play crucial roles in determining the major product.
How can HBr peroxides be utilized in practical applications?
HBr peroxides find applications in polymer synthesis, pharmaceutical manufacturing, and various industrial processes.